INTRODUCTION
Soursop (Annona muricata L.), also called “guanábana” in some countries of South America, is a species of the Annonaceae family. Its center of geographic diversity is in the north of South America and it is distributed in different tropical regions of the world (Love and Paull, 2011). This fruit is highly appreciated due its excellent flavor and nutraceutical properties. In addition, some therapeutic effects are related to fight against cancer cells (Zorofchian et al., 2015; Jemimah et al., 2016). For that reasons, the markets of the USA, Europe and Asia demand this appetizing fruit (Pro Ecuador, 2014).
In Ecuador, soursop cultivation is a growing business and offers great opportunities due to there is big demand and fruit obtain high prices (Moreira and Héctor, 2014). Currently an area of 800 ha is estimated for this crop, although official data are not available. (Personal information obtained from the Fruticulture Program of INIAP, Litoral Sur Experimental Station.
Morphological characterization of the genetic resources of several species of Annonaceae has been of transcendental importance to register particular traits and to differentiate their taxonomic, phenotypic and genetic through qualitative and quantitative attributes that are highly heritable and observable, as well as expressible in most of the environments (Suratman, and Mulyani, 2015). The systematic characterization has allowed to reveal the variation within the collections and select the most elite genotypes for their cultivation.
Fruit physico-chemical characteristics (size, shape, types of emergencies, concentration of total soluble solids, acidity and others) have been investigated in the central littoral of Ecuador (Moreira et al., 2016), however the phenotypic features of the plant are not have been studied, Therefore, the principal goal of this study was to determine the morpho-agronomic characteristics of a soursop population, established wildly in the province of Manabí, Ecuador.
MATERIALS AND METHODS
Germplasm location
The research was carried out in an area of 1273.45 km2 distributed between the cantons Jipijapa, Paján, 24 de Mayo and Olmedo in the south of Manabí, Ecuador. This zone has a dry tropical climate with an average temperature of 25.85 °C, 81.45 % of relative humidity and 1484 mm of annual accumulated precipitation (INAMHI, 2014). For this study, 60 soursop trees (accessions) in productive stage were chosen (Table 1).
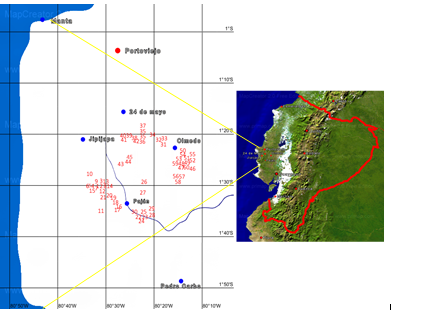
Prior to the morphoagronomic characterization of the accessions, the location and distribution of the accessions in the study area was determined, through the Global Positioning System (GPS), with a Garmin® GPS 12 model. Latitude, longitude and altitude above sea level were determined. Subsequently, with the database obtained, the geographical distribution of the accessions on a satellite map was graphed with the use of MAPCREATOR v.19 software, in order to register and visualize its geospatial distribution (Table.1).
Table 1. Population of soursop selected from southern of Manabí, Ecuador.
Table 1-
Population of soursop selected from southern of Manabí Ecuador
Figure 1:
Global geographical positioning (GPS) of 60 accssesion of the soursop germplasm in southern of Manbí, Ecuador.
Characterization
The morphological characterization was carried out using 17 plant descriptors (Bioversity International y CHERLA, 2008), being 9 quantitative (number of fruits per tree, diameter of the canopy (m), tree height (m), number of nodes per meter of branch, leaf length (cm), leaf width (cm), petiole length (cm), petiole thickness (mm), and number of primary leaf veins) and 10 qualitative (trunk color, young branch color, trunk ramification, leaf shape, leaf base shape, leaf apex shape, color of the mature leaves, leaf margin, fruit shape and form of emergencies of the exocarp). Ten observations per tree (accession) were made for each trait, with the exception of the number of nodes per meter of branch in which five observations were measured per tree. These evaluations were carried out during the dry period of the year, which coincides with the fruiting stage of the tree.
Analyses
In terms of quantitative descriptors, a descriptive analysis was made (mean, standard deviation, minimum value, maximum value and variation coefficient), as well as Pearson correlations. In terms of qualitative descriptors, a frequency analysis was carried out and the results were expressed in percentages. Principal component analysis (PCA) was made based on the Pearson correlation matrix selecting descriptors that showing greatest contribution in the morphological characterization. The selection criteria of eigenvectors involved the values closest to the highest value and the percentage contribution of each axis to the total variability.
A cluster analysis was performed to achieve the grouping of individuals according to their similarities, including quantitative and qualitative characteristics through the Gower distance matrix and the Ward method as a form of ascending hierarchical aggregation. A discriminant analysis was performed to verify the correspondence of the accessions within the dendrogram conglomerates, the statistical data analyses was made using the INFOStat software (Di Rienzo et al., 2016, free version).
Each tree of the wild studied population was considered as an accession, in which the age of the trees was variable,; however it ensured that all of them were of productive age.
RESULTS AND DISCUSSION
Five descriptors (Number of fruits, crown diameter, tree height, leaf petiole length and thickness of leaf petiole) showed coefficient of variation values above 20 %, which is considered by Franco and Hidalgo (2003) as the minimum limit for the expression of variability (Table 2). These authors consider that descriptors with values lower than 20 % should be considered as low variability; in this study the descriptors that did not reach this level were nodes per meter of branch, length of leaf, leaf width and number of primary veins per leaf. Number of fruits was the descriptor that showed the greatest variability (CV: 60, 11). In conditions of commercial cultivation, the amount of fruits per tree is mainly due to the management of the plantation plus the genetic constitution of the trees. However, the locations where the germplasm was studied have the same climate, and the trees don’t receive any cultivation work. Therefore, it woud be that the number of fruits per tree would be due to the genetic structure of each accession. This character is particularly important because is related to productivity and profitability, being priority in genetic improvement programs (Jameel et al, 2015).
Table 2-
Quantitative morphological descriptors of the 60 soursop accessions characterized in situ in southern of Manabí
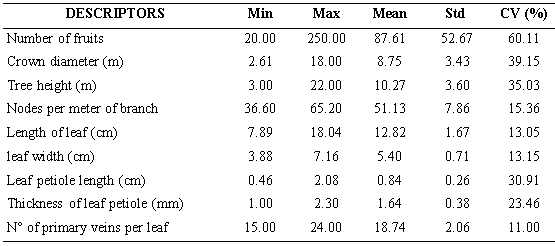
Based on the considerations about the variability expressed by Franco and Hidalgo (2003) and Hernández (2013), it can be suggested, that there is greater variability of the Ecuadorian soursop germplasm compared to the germplasm studied by Padmini et al. (2013), in which 448 soursop accessions were characterized, discarded the length of the leaf, the length of the petiole of the leaf (due to low variation coefficient), the shape of the apex and the base of the leaf (in both case only one form), the margin of the leaf (entire 100 %), and the color of the mature leaf (dark green 100 %), characters that were highly variable in the Ecuadorian germplasm.
No significant correlation coefficients were observed between the morphological descriptors, except between the number of fruits and the canopy diameter, which showed a highly significant positive correlation, although with a medium coefficient (r = 0.54). Denoting the implication and importance of the number of fruit in the productivity. It is necessary to carry out more research because this result suggests that trees with a greater diameter of the canopy are more productive in terms of number of fruits than those of vertical growth. As this study was development in wild trees that do not receive any cultivation work, this information would be very valuable in breeding programs.
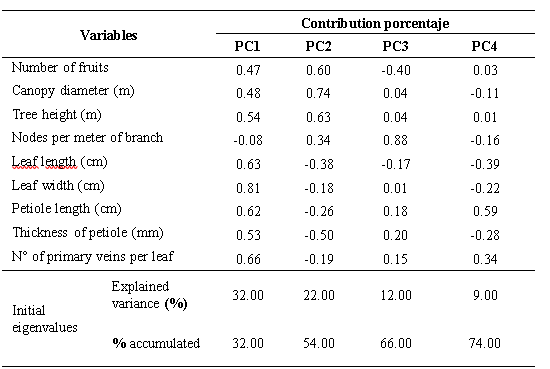
The analysis of main components allowed us to illustrate the relationship between the quantitative variables studied and their participation in the explanation of germplasm variability. The contribution percentage of the first four components was 74 % of the total variability. The first component (PC1) contributed with 32 % of the variability, followed by the second component (PC2) with 22 %, the third component contributed with 12 % and the fourth 9 %, respectively (Table 3). In the PC1, the descriptors that contributed most to the variability were: leaf width, number of primary veins per leaf, leaf length and petiole length variables. The mentioned variables are related to the uptake of sun energy that is a determining factor in the photosynthetic activity and photoassimilates flow (Meza y Bautista, 1999). In the PC2, the descriptors that contributed most to the variability were: Number of fruits, Canopy diameter and tree height, variables that determine the productivity and structure of the tree.In Colombia, Miranda et al. (2000) observed the formation of five groups and reported only oblong-lanceolate leaves with acute apices, a characteristic that denotes a lower variability of this germplasm in situ with respect to that observed in southern Manabi, in which four types appeared of leaves in terms of their shape and three different types of apices. These authors also observed leaves with average lengths of 12.00 cm and 4.78 cm wide, while in Manabí average values greater than 12.88 cm and 5.45 cm, respectively were observed. The aforementioned authors did not use the descriptors used in the present investigation, where the length and width of the leaf petiole, the number of ribs on the leaf and the type of undulation of the leaf were discriminant.In base to the expressed by Franco and Hidalgo (2003) and Hernández (2013), about the variability we can suggest the existence of greater variability of the Ecuadorian soursop germplasm versus other germplasms such as the studied by Padmini et al. (2013), who in the characterization of a germplasm of 448 soursop accessions in Sri Lanka, Asia, with the use of 45 characters, discarded the length of the leaf, the length of the petiole of the leaf, the shape of the apex and of the base of the leaf, the undulation of the leaf, and the color of the mature leaf, characters that instead were highly variable in the Ecuadorian germplasm.
Table 3-
Variability explained in the PCA for the quantitative morphological descriptors.
The hierarchical cluster analysis of Ward allowed the conformation of three similarity groups (Fig. 2), which expressed a cofenetic correlation of 0.57, which represents that there is adequate reliability in the analyzes.
Figure 2:
Dendrogram obtained by Ward's hierarchical grouping of the quantitative and qualitative morphological descriptors of 60 in situ germplasm accessions of soursop from the central regions of ecuadorian littoral. Cofenetics correlation r = 0.57
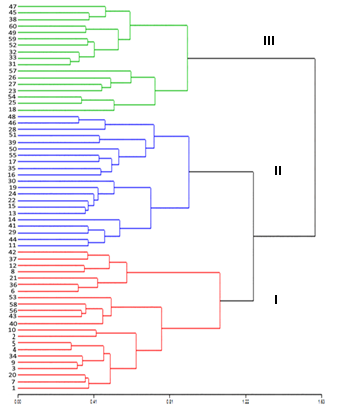
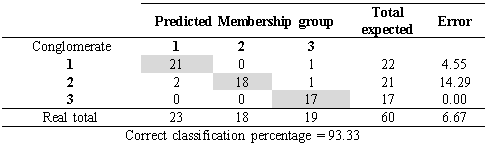
The discriminant analysis showed that only 6.7 %, corresponding to four accessions, had an incorrect classification, that is to say, they presented more similarities with plants from other conglomerates than with those of their own. On the other hand, 93.34 % of the accessions (56 of 60) presented an adequate classification, which indicates that they corresponded to the right conglomerate (Table 4). The group 1 consisted of 22 accessions, group 2 of 21 accessions and group 3 of 17 accessions.
Table 4
Cross classification apparent error of the clusters by discriminant analysis of the morphoagronomic descriptors from the in situ germplasm accessions of soursop from the central regions of the ecuadorian Litoral
Conglomerate 1 contains the accessions showing the lowest average number of fruits (57.23), the smallest diameter of the crown (7.03 m) and lowest height (8.51 m). They presented average values of magnitude of the leaf in comparison to the other conglomerates, as well as the lowest number of nodes per meter of branch (50.14). conglomerate 2 included accessions showing the largest number of fruit (121.71) and with the largest canopy diameter (11.70 m) and largest height (12.67 m). It also grouped the accessions with the highest values in terms of the descriptors that determine the magnitude and carrying capacity of sap of the leaf such as leaf length and number of primary veins per leaf. The conglomerate 3 integrated the accessions showing an intermediate amount of number of fruits (84.82), number of nodes per branch (53.20), number of ribs per leaf (17.54), lowest values of leaf length (11.66 cm) and leaf width (4.88 cm).
The present study described six types of trunk coloration and the young branch, four types of shape leaf, two types of the leaf base, three types of the leaf apex, three colorations of the mature leaf and three types of the margin of the leaf blade (Table 5).
Regarding the expression of the qualitative descriptors in the conglomerates conformation, differences were observed in the color of the trunk. Conglomerates 1 and 3 presented mainly greenish-brown color (31.81 % and 35.29 %, respectively), while conglomerate 2 showed dark brown color (28.57 %). The color of the young branch showed a predominance of light brown in the conglomerates 1, 2 (63.64 %, 38.09 %, respectively). Conglomerate 3 had trees with greenish-brown branches color (35.29 %). Trees conformed by a single main stem were in all conglomerates (68.18 %, 61.90 % and 36.29 % in the conglomerates 1, 2 and 3, respectively). Lanceolate shape of leaf was predominant in conglomerates 1 and 3 (95.45 % and 70.59 %, respectively); while conglomerate 2 had less percentage (47.62 %). The acute form of the leaf base was observed in high percentage in all conglomerates (90.91 % conglomerate 1, 100 % conglomerate 2 and 70.59 % conglomerate 3).
Table 5-
Qualitative morphological descriptors of 60 accessions in situ of soursop germplasm from southern of Manabí, Ecuafor.
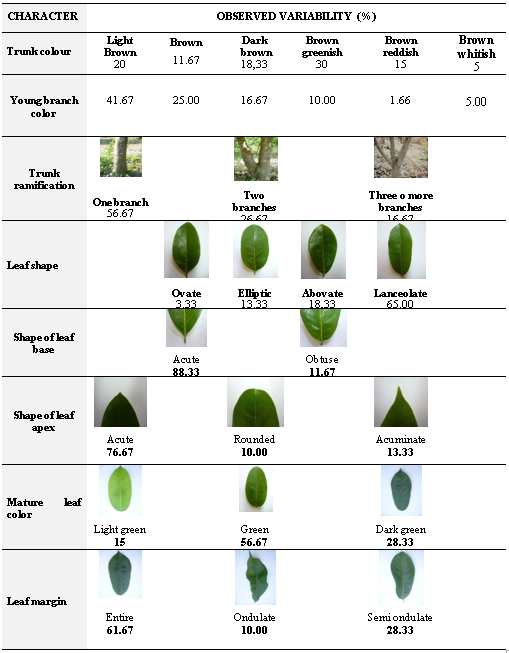
Despite the major features presented, different frequencies of the qualitative descriptors in the three conglomerates studied constitute an evidence of the existence of phenotypic variability in the soursop germplasm that was in situ.characterized.
Miranda et al. (2000) in study development in Colombia, observed the formation of five groups using the conglomerate analyses. These authors reported only the presence of oblong-lanceolate shape of leaves with acute apex, denoting a lower variability respect to the variability observed in southern of Manabí which showed four types of leave shape and three types of apex. The results of this research partially coincide with the results obtained by Constance et al. (2015), who found that the length and width of the leaf and number of fruits were determinants to reveal the variability in 42 soursop accessions.
The lanceolate leaf shape was predominant in this study, coinciding with Castañeda (2014), who found the same predominate shape in the Annonaceae species. The presence of ondulate and semi ondulate leaves in these study, was also observed, as well as, four types of leaf shape, two types of leaf base shape, three types of leaf apex shape, and three mature leaf colors.
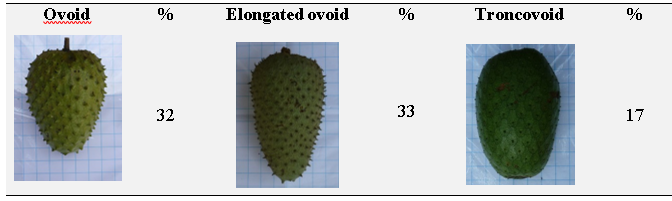
The most frequent forms of the fruit in the evaluated germplasm were the elongated ovoid and the ovoid, followed by the troncovoid type (Table 6). The rest were distributed among cordiform, spherical and reniform fruits.
Table 6-
Predominant forms of the fruits of in situ soursop germplasm from southern of Manabí, Ecuador.
The studied germplasm presented several forms of spines of the exocarp, of which the most common one was the acute apex of up to 5 mm in length and not very dense in the fruit (58 %) (Table 7). This form was followed by those in the form of stingers, very dense in the fruit and with a length up to 10 mm (18 %) and the spines erect with acute apex and rounded at its base with a length up to 2 mm (17 %). The form of spines could be linked to specific genetic structures, so complementary research on this subject should be initiated.
Table 7
Forms of spines of the exocarp that predominate in the in situ soursop germplasm from southern of Manabí Ecuador
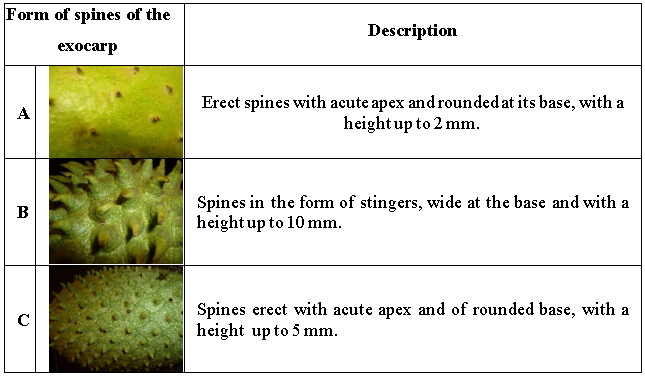
In general, there are few studies about the characteristics of the exocarp spines and the soursop fruit forms that provide precise details and definitions about them (Moreira et al., 2016). Spines are simply called "spine" and the fruits in most cases it is attributed a cordiform, oval and conical aspect (Benavides, 1997, Miranda et al., 2000, Pinto et al., 2005).









